How Kareo Discovered 10% of the Local Population May Have Contracted COVID-19
This past weekend I partnered with Dr. Jennifer Armstrong, M.D. and organized a COVID-19 drive thru antibody test for 415 employees and guests and I’m writing to share what we learned from this incredible experience.
A few weeks ago, Mark Cuban was quoted as saying that how companies treat their workers during the COVID-19 pandemic could define their brand for decades. I couldn’t agree more.
Given the disruption and uncertainty we have all faced, and the lack of clarity on what comes next, we’ve taken a series of actions to start to deliver peace of mind to our employees. We moved all employees to work from home before “stay at home” orders were issued. We started weekly virtual townhall meetings to share real-time business metrics about how the pandemic was impacting our company. We ordered surgical masks and hand sanitizers to be delivered to our employees. We recognized employees that have front-line healthcare workers in their family and delivered dinners to their homes.
The latest thing we’ve done was organizing a COVID-19 drive thru antibody test this past weekend. We did this to show our employees that we care about their health and safety, but we also wanted to learn more about how a return to work might be managed at some point in the future.
And learn we did! Most notably that the apparent simple act of testing is far more complex, requiring difficult decisions and planning around which tests to use, test sourcing and delivery logistics, test subject communication and follow-up, and policy considerations driven by individual and population test results.
I’m a software entrepreneur, not a virologist. As such, I don’t claim to be an expert on the dynamic and rapidly changing science of COVID-19 testing. But I’d like to share a few things I’ve learned in the past couple weeks.
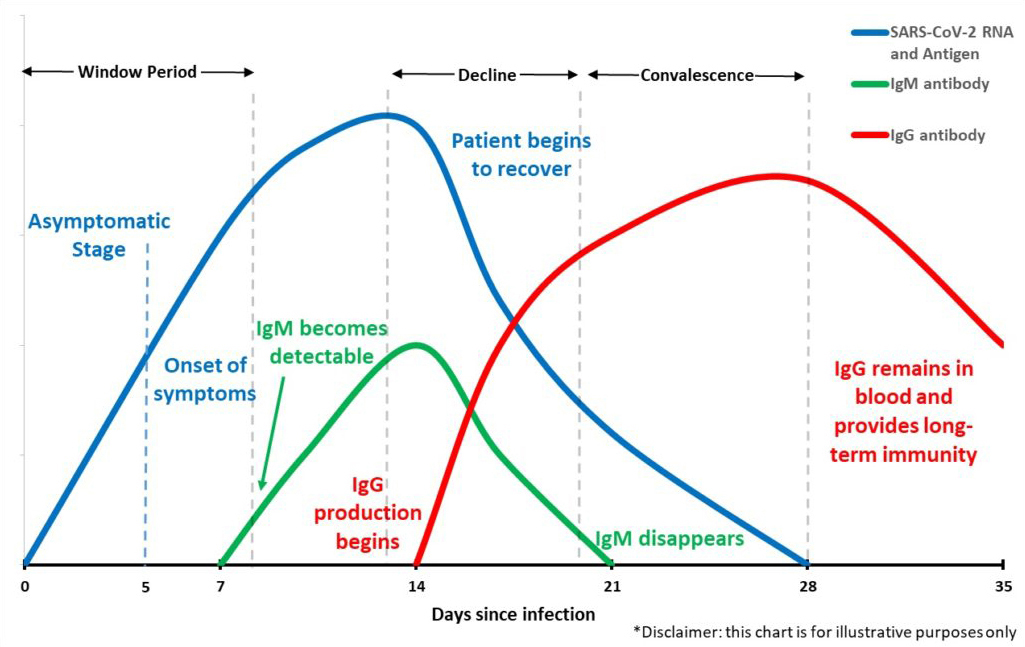
There are two main types of tests for COVID-19; molecular and serological.
Molecular tests (referred to as “PCR tests”) detect genetic information of the virus, like DNA or RNA. This is only possible if someone is actively infected with the virus. These tests are conducted by collecting nasal swab samples that are sent to laboratories where results are returned typically within a few days. This type of test detects the virus typically 5-7 days before antibodies start to form in the body.
Serological tests (referred to as “Antibody tests”) detect antibodies in your blood which are specific proteins made by your immune system in response to infections. These tests are conducted by collecting blood samples and detecting the existence of antibodies to a specific viral strain. The blood samples can be tested by a lab or by using rapid test kits that now produce results in as little as 15-20 minutes. This type of test detects the virus only after the body begins producing antibodies, which usually occurs 5-7 days after the body is infected.
To truly understand where a patient may be along the lifecycle of a COVID-19 infection, you would need to perform both tests. This would tell you if you are a) positive or negative for the virus, b) positive and fighting an active infection, or c) recovered and possibly have immunity.
We decided to approach our employee testing as a screening test and company research effort, not a diagnostic test upon which our employees should make specific healthcare decisions. We wanted to explore questions around how many people may have contracted the virus and learn more about the logistical realities of what returning to work might look like. For these reasons, we decided to administer a COVID-19 antibody test capable of detecting both IgM and IgG antibodies in the body. The tests were completely voluntary.
Sourcing the Antibody TestsTo secure antibody tests, we partnered with Dr. Jennifer Armstrong, M.D., a board-certified physician and surgeon who owns an independent healthcare practice in Newport Beach, California. Dr. Armstrong has a complex laboratory license and is authorized to buy and administer various types of tests.
For safety and efficiency reasons, we decided to pursue drive thru testing and explore using one of the many rapid antibody test kits that are just starting to hit the market. The FDA allows such tests for public health surveillance to gain greater clarity on actual infection rates. Dr. Armstrong spoke with colleagues at leading healthcare systems and selected a rapid antibody test kit which reports a 96.46% sensitivity and 97.4% specificity and has been registered with the FDA under Section IV.D of the FDA’s Policy for Diagnostic Tests for Coronavirus.
Since we were trying to understand how a program like this might help get people back to work, not perform diagnostic testing, the accuracy was not the most important factor. We were aware that no test is 100% accurate and we believe COVID-19 antibody testing will get better over time.
Kareo’s Drive Thru Testing ExperimentTo administer the tests, we decided to arrange drive thru testing over the weekend in the parking lot outside Dr. Armstrong’s office supported by her existing medical staff along with an administrative team provided by Kareo.
Prior to the test date, we invited employees and their guests to participate in the test which we made very clear was completely optional. Each subject was asked to complete an online form with basic demographics and a preferred arrival time. We used this information to build a schedule with subjects arriving every 5-10 minutes and assigned to one of three drive thru testing lanes. Subjects were asked to print and sign a couple of forms, including a form that provided the proper disclosures and follow up protocol as required by the FDA, and bring the forms with them to the test site.
On the day of the test, subjects arrived in their cars with masks covering their faces. The administrative team checked subjects in at the parking lot entrance and routed them to their designated testing lane. The medical team pricked each subject’s finger to collect and apply a blood sample to a rapid test kit, handed the test kit back to the subject, and asked them to drive their car to a designated parking spot and wait 20 minutes. Then, Dr. Armstrong or her physician’s assistant visited each subject’s car to read and capture their test results in a chart. Finally, after receiving their results, subjects drove home from the site.
We learned several important best practices to ensure safe and effective testing:
- Subjects should be educated about the tests ahead of time
- All administrative and medical staff should be tested before testing subjects
- Personal protective equipment should be worn by all administrative and medical staff
- Subjects should stay in their cars for the duration of their visit to the site
- Privacy of specific test results should be strictly maintained throughout the test
After the drive thru testing, we sent all subjects an email to remind them that these tests like many others on the market are not 100% accurate and they should review the results of their test with their personal doctor to understand the results and the proper course of treatment. We also encouraged subjects that regardless of their test results, to follow the recommendations of the CDC, including:
- Maintain social distancing.
- Stay home as much as possible.
- Wash your hands often with soap and water for at least 20 seconds especially after you have been in public places or after blowing your nose, coughing or sneezing.
- Clean and disinfect frequently touched surfaces daily.
- If you feel sick seek medical attention.



The COVID-19 antibody tests deliver four possible results:
- No antibodies are present (indicated by a “C” marker)
- Antibodies are present, no active infection and possible immunity (indicated by an “IgG” marker)
- Antibodies are present with a possible active infection (indicated by an “IgM” marker)
- Antibodies are present with a possible active infection and are in the process of developing long term immunity (indicated by an “IgM” marker as well as “IgG” marker)
To maintain strict patient privacy, Dr. Armstrong and her team did not share individual test results with Kareo. But the aggregate tests results were reported as follows:
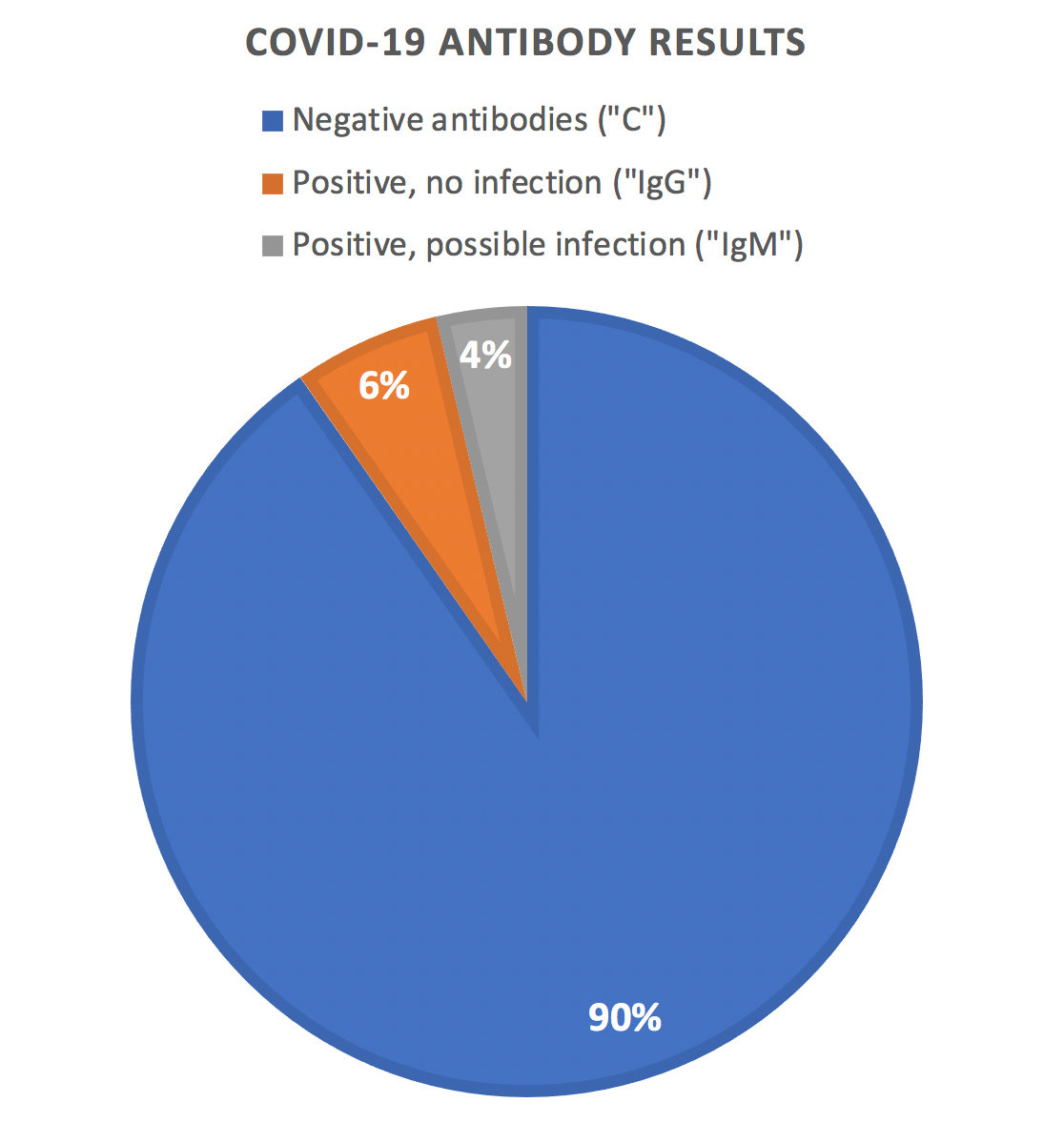
- 415 unique subjects tested
- 375 (or 90.3%) tested negative for antibodies (“C”)
- 40 (or 9.63%) tested positive for antibodies (“IgG” or “IgM”)
- 25 (or 6.0%) tested positive for antibodies with no active infection (“IgG”)
- 15 (or 3.6%) tested positive for antibodies with possible active infection (“IgM”)
Of the subjects that tested “IgG”, 22 of 25 (or 88%) of the subjects remember being sick in the past 8 weeks. And of the subjects that tested “IgM”, only one reported any symptoms at the time of the test.
Finally, any subject that tested “IgM” was referred to another healthcare provider for a molecular (“PCR”) test and treatment and recommended to be placed into home isolation per standard protocol. All other subjects were recommended to continue to follow “stay at home” orders and practice social distancing regardless of their test results.
Overwhelming Response from Kareo EmployeesKareo has received overwhelmingly positive response from its employees on the series of actions we’ve taken to demonstrate that we care about their health and safety, including our COVID-19 antibody testing.
Here is just a small sampling of emails that I have received from Kareo employees over the past week:
- “I’ve been incredibly impressed by how far above and beyond Kareo has gone during this pandemic. You guys are handling it really well.”
- “First off, WOW! This is amazing and I thank you and everyone that was involved in making this happen. It’s things like this that makes me proud to work for Kareo! Thank you for taking care of your employees.”
- “We are extremely fortunate to work for a company like Kareo and then to receive resources like this just takes it to the next level. I am proud to be a member of this organization and thankful for a leadership team who truly puts our health and safety first.”
- “Just wanted to send you over a quick note to express gratitude in everything you and the organization are doing in response to COVID-19. I’m sure you’ve received plenty of these emails, but I wanted to personally thank you. I’ve been with Kareo a long time and this is a great example of why.”
- “A sincere thank you for all of this. I appreciate how well you’ve communicated and demonstrated that the employees here are valued and important. Thank you SO much.”
So, where do we go from here? To be honest, like everything we’ve experienced with COVID-19, we’re still figuring that out. But we do have a few ideas that seem worth exploring.
First, we know that the science and technology of COVID-19 testing is rapidly improving and evolving. Testing is going to look completely different in one month, three months, and six months from now. So, we will consider offering new and improved tests with even greater precision to our employees as they become available.
Second, we’ve received tremendous interest from other business leaders in the community and we are interested in collaborating with them and playing some role in helping to scale COVID-19 testing within our local community and pool resources to develop better data and analytics about the spread and the prevalence of potential immunity to the virus.
Finally, Kareo is in a unique position as a leading supplier of cloud-based software to more than 75,000 healthcare providers in independent medical practice throughout the United States. We’re interested in sharing our experience with our customers and challenging them to help solve the testing needs in this country. For more resources available to help navigate the COVID-19 crisis, visit our COVID-19 page here.
If you are interested in getting involved, please contact me at [email protected].
















